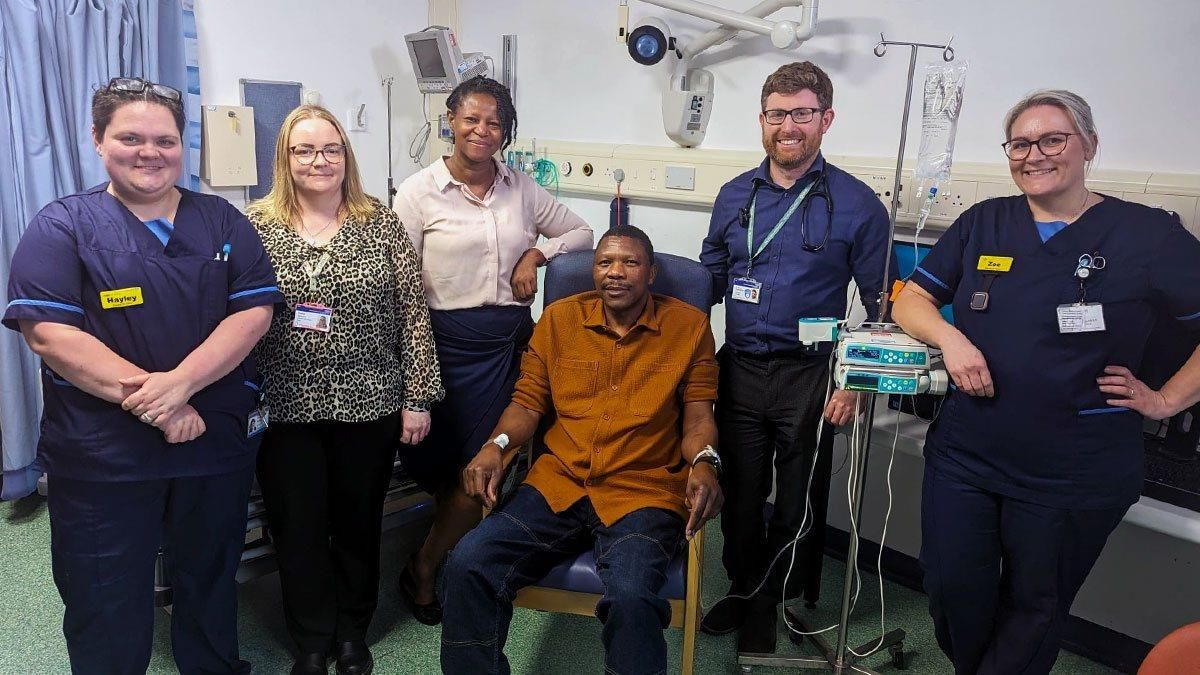
The Queen Elizabeth Hospital Birmingham (QEHB), operated by BHP founder-member University Hospitals Birmingham NHS Foundation Trust, has treated its first patient in England with a personalised vaccine against their bowel cancer, in a clinical trial which is part of NHS England’s new Cancer Vaccine Launch Pad (CVLP).
In a national first, father-of-four Elliot Pfebve received the developmental jab within the Clinical Research Facility at QEHB, one of several sites taking part in the colorectal cancer vaccine trial sponsored by BioNTech SE.
The trial is one of several that will be taking place in NHS trusts across the country to treat different types of cancer. Thousands more patients are expected to benefit from NHS England’s new CVLP, which will enable those wanting to participate in clinical trials to be fast-tracked to one of the nearest participating hospitals.
Patients who agree to take part have a sample of their cancer tissue and a blood test taken. If they meet a clinical trial’s eligibility criteria, they can be referred to their nearest participating NHS site, meaning patients from hospitals across the country will find it easier than ever to take part in groundbreaking research.
The investigational cancer vaccines evaluated in the colorectal cancer trial are based on mRNA – the same technology used for the Pfizer-BioNTech COVID-19 vaccine – and are created by analysing a patient’s tumour to identify mutations specific to their own cancer. Using this information, medics then create an experimental individualised cancer vaccine.
The developmental vaccines are designed to induce an immune response that may prevent cancer from returning after surgery on the primary tumour, by stimulating the patient’s immune system to specifically recognise and potentially destroy any remaining cancer cells.
The investigational cancer vaccines being jointly developed by biopharmaceutical companies BioNTech and Genentech, a member of the Roche Group, are still undergoing trials and have not yet been approved by regulators.
Higher-education lecturer Elliot, 55, had no cancer symptoms and was diagnosed through a routine health check with his GP.
A CT scan and a colonoscopy confirmed he had colon cancer and Eliott had surgery to remove the tumour and 30cm of his large intestine. He was then referred to the QEHB for initial rounds of chemotherapy and to take part in a clinical trial.
Eliott said: “Taking part in this trial tallies with my profession as a lecturer, and as a community-centred person. I want to impact other people’s lives positively and help them realise their potential.
“Through the potential of this trial, if it is successful, it may help thousands, if not millions of people, so they can have hope, and may not experience all I have gone through. I hope this will help other people.”
Thirty hospitals in England are already signed up to the pioneering Cancer Vaccine Launch Pad – one of the biggest projects of its kind in the world – with more sites joining the platform over the coming months.
The scheme aims to expand and work with a range of partners in the pharmaceutical industry to include patients across many cancer types who could potentially join a vaccine trial, such as those with pancreatic and lung cancer.
Principal Investigator for the trial at QEHB, Consultant Clinical Oncologist, Dr Victoria Kunene, said: “The investigational cancer vaccines are based on mRNA and are created by analysing a patient’s tumour to identify mutations specific to their own cancer. Using this information, we can create an individualised investigational cancer vaccine, but it is too early yet to say if these will be successful, though we are extremely hopeful.
“Based on the limited data we currently have of the in-body response to the vaccine, this could prove to be a significant and positive development for patients, but more data is yet needed and we continue to recruit suitable patients to the trial to establish this further.”
Amanda Pritchard, NHS chief executive, said: “Seeing Elliot receive his first treatment as part of the Cancer Vaccine Launch Pad is a landmark moment for patients and the health service as we seek to develop better and more effective ways to stop this disease.
“Thanks to advances in care and treatment, cancer survival is at an all-time high in this country, but these vaccine trials could one day offer us a way of vaccinating people against their own cancer to help save more lives.
“The NHS is in a unique position to deliver this kind of world-leading research at size and scale, and as more of these trials get up and running at hospitals across the country, our national match-making service will ensure as many eligible patients as possible get the opportunity to access them.”
Trials have already enlisted dozens of patients, although the majority of participants are expected to be enrolled from 2026 onwards.
Professor Peter Johnson, NHS national clinical director for cancer at the NHS said: “We know that even after a successful operation, cancers can sometimes return because a few cancer cells are left in the body, but using a vaccine to target those remaining cells may be a way to stop this happening.
“Access to clinical trials could provide another option for patients and their families, and I’m delighted that through our national launch pad we will be widening the opportunities to be part of these trials for many more people, with thousands of patients expected to be recruited in the next year.”
Executive Director of Research and Innovation at Cancer Research UK, Iain Foulkes, said: “It’s incredibly exciting that patients in England are beginning to access personalised cancer vaccines for bowel cancer.
“This technology pioneers the use of mRNA-based vaccines to sensitise people’s immune system and in turn detect and target cancer at its earliest stages. Clinical trials like this are vital in helping more people live longer, better lives, free from the fear of cancer. If successful, the vaccine will be a game changer in preventing the onset or return of bowel cancer.”
Last year, the Government signed an agreement with BioNTech to provide up to 10,000 patients with precision cancer immunotherapies by 2030.
BioNTech has already begun conducting clinical trials in the UK, and the NHS launch pad is helping to accelerate the identification of eligible patients for those trials in England.
The vaccines being tested as part of the trials aim to help patients with different types of cancer and, if successfully developed, researched and approved, cancer vaccines could become part of standard care.
The NHS is working in partnership with Genomics England on the launch pad, with work already helping patients access the latest testing technologies and ensures they are given more targeted precision treatments for their cancer.
You might also be interested in: